
InnovaMatrix® AC Placental Extracellular Matrix
Premium Product. Optimal Economics.
InnovaMatrix® AC is available for use in your office and wound care clinic.
 InnovaMatrix® AC is an important and innovative advancement in the treatment of wounds and is available for office use with the HCPCS code A2001. InnovaMatrix® AC is the first-ever placental-derived medical device cleared by the FDA for wound management.
InnovaMatrix® AC is an important and innovative advancement in the treatment of wounds and is available for office use with the HCPCS code A2001. InnovaMatrix® AC is the first-ever placental-derived medical device cleared by the FDA for wound management.
Premium Product
As a new category of products, InnovaMatrix® AC offers the inherent benefits of the placenta1,2 plus the quality control, reliability, and safety profile of a medical device3,4.
Optimal Economics
InnovaMatrix® AC offers a wider variety of sizes with optimal economics for different sites of care.
Contact Us
Learn more about how InnovaMatrix® AC can deliver innovative, medical device quality and performance to your patients, and optimal economics to your bottom line.

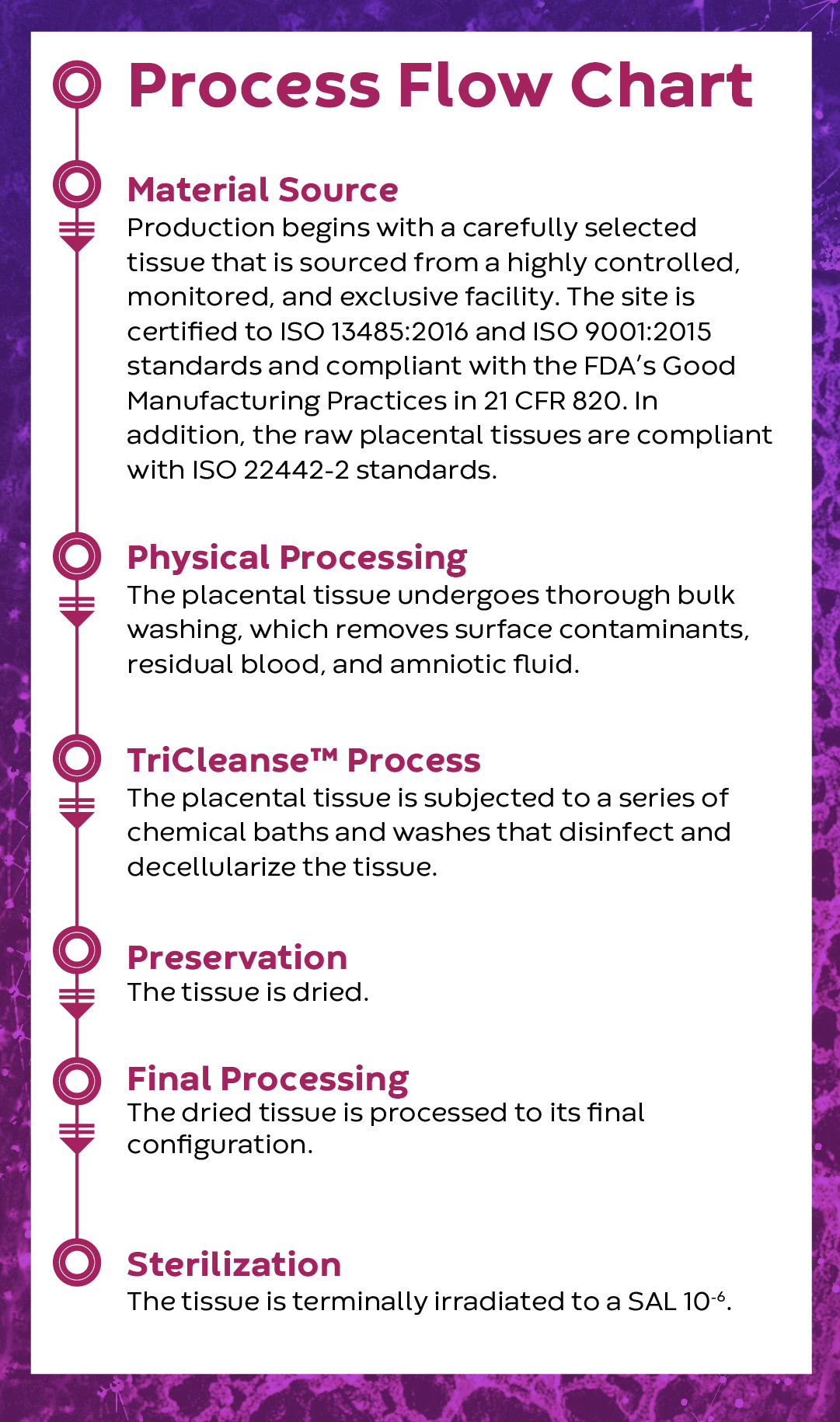
Material Selection
While the benefits of placental-derived allografts are well documented1,2, human-derived grafts can be extremely variable due to the unique medical histories and social behaviors of each donor5-12. Knowing this challenge, a porcine source was selected because it can be controlled for age, diet, health, and activity level, thereby greatly reducing the variability of the raw material used to manufacture InnovaMatrix® AC.
The TriCleanse™ Process balances the need for decellularization while maintaining structural proteins of the ECM.3,4,16,17
A Critical BalanceEffective decellularization removes cells and cellular debris while maintaining the structural proteins of the ECM18. |
 |
|---|

A Critical Balance
Effective decellularization removes cells and cellular debris while maintaining the structural proteins of the ECM11.
The presence of cells and or cellular debris in biologic wound dressings can cause an inflammatory reaction resulting in a greater M1:M2 ratio macrophage response by the host immune system at the wound site. Thoroughly decellularized biologic skin scaffolds allow the host to react directly with a healing response, generally associated with a greater M2:M1 ratio of macrophages and leading to collagen deposition19,20.

Balancing Decellularization Efficiency and the Denaturing of ECM Proteins
As a placental medical device processed with the proprietary TriCleanse™ Process, InnovaMatrix® AC was extensively biochemically characterized to identify and quantify the different structural and functional proteins. These results were then compared to an SIS membrane device as a base line for comparison.
Cellular Debris Comparison4

The presence of intact cells and nuclei creates an immunogenic response as cellular antigens are recognized as foreign by the host’s immune system.

The immune system has the innate capability to recognize nucleic acids not contained within the nucleus via pattern recognition receptors and mount an inflammatory response to their presence.
Hematoxylin and Eosin Staining4
Hematoxylin and Eosin (H&E) staining is commonly used to stain tissue for histology evaluation21. H&E stains nuclei blue and extracellular matrix and cytoplasm pink and other tissues shades in between.
InnovaMatrix®

The H&E staining shows a minute quantity of residual nuclei in the finished product.
SIS Commercial Membrane

The H&E staining shows clearly stained blue nuclei are distributed throughout the finished product.
HCT/P Commercial Graft

The H&E staining shows blue stained nuclei present in the entirety of the graft with areas of high concentration.
First-ever placental-derived medical device cleared by the FDA for wound management


Summary of Indications, Contraindications, and Warnings
Indications
InnovaMatrix® AC is indicated for the management of wounds including:
- Partial and full-thickness wounds
- Pressure ulcers
- Venous ulcers
- Diabetic ulcers
- Chronic vascular ulcers
- Tunneled/undermined wounds
- Surgical wounds (donor sites/grafts, post-Mohs surgery, post- laser surgery, podiatric, wound dehiscence)
- Trauma wounds (abrasions, lacerations, and skin tears)
- Partial-thickness second degree burns
- Draining wounds
This device is intended for one-time use.
Contraindications
This device is derived from porcine collagen and should not be used on patients with sensitivity or allergy to porcine materials; sensitivity or allergy to collagen; or active or latent infection in or around the application site.
This device is not indicated for use in third degree burns.
Disclaimer
This information is not an affirmative instruction as to which codes and modifiers to use for a particular service, supply, procedure or treatment. It is the provider’s responsibility to determine and submit the appropriate codes and modifiers for any service, supply, procedure or treatment rendered. Actual codes and/or modifiers used are at the sole discretion of the treating physician and/or facility. Local payers and physician specialty societies should be contacted for specific coding guidelines. Convatec cannot guarantee medical benefit coverage or reimbursement with the codes listed here.

Download our
InnovaMatrix® AC Documents
Use the arrows to navigate.
Available Sizing
| Size | Product Number |
|---|---|
| 15 mm Disc | IMX-15MM-01 |
| 2 cm x 2 cm | IMX-0202-01 |
| 4 cm x 4 cm | IMX-0404-01 |
| 4 cm x 6 cm | IMX-0406-01 |
| 5 cm x 5 cm | IMX-0505-01 |

MKT-2023-0051-18E V01
- Shaifur Ra, M., Islam, R., Asaduzzama, S.M., & Shahedur R, M. (2015). Properties and Therapeutic Potential of Human Amniotic Membrane. Asian Journal of Dermatology, 7(1), 1-12. doi: 10.3923/ajd.2015.1.12
- Fairbairn, N. G., Randolph, M. A., & Redmond, R. W. (2014). The clinical applications of human amnion in plastic surgery. Journal of Plastic, Reconstructive & Aesthetic Surgery, 67(5), 662-675.
- K193552 510(k) Summary
- Data on file -RDR-002
- Cardinal, L. J. (2015). Central tendency and variability in biological systems. J Community Hosp Intern Med Perspect, 5(3), 27930. doi:10.3402/jchimp.v5.27930
- Collier, A. C., Tingle, M. D., Paxton, J. W., Mitchell, M. D., & Keelan, J. A. (2002). Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum Reprod, 17(10), 2564-2572. doi:10.1093/humrep/17.10.2564
- DuBois, B. N., O’Tierney-Ginn, P., Pearson, J., Friedman, J. E., Thornburg, K., & Cherala, G. (2012). Maternal obesity alters feto-placental cytochrome P4501A1 activity. Placenta, 33(12), 1045-1051. doi:10.1016/j.placenta.2012.09.008
- Huuskonen, P., Amezaga, M. R., Bellingham, M., Jones, L. H., Storvik, M., Hakkinen, M., . . . Pasanen, M. (2016). The human placental proteome is affected by maternal smoking. Reprod Toxicol, 63, 22-31. doi:10.1016/j.reprotox.2016.05.009
- McRobie, D. J., Glover, D. D., & Tracy, T. S. (1998). Effects of gestational and overt diabetes on human placental cytochromes P450 and glutathione S-transferase. Drug Metab Dispos, 26(4), 367-371. Retrieved from https://www.ncbi.nlm. nih.gov/pubmed/9531526
- O’Huallachain, M., Karczewski, K. J., Weissman, S. M., Urban, A. E., & Snyder, M. P. (2012). Extensive genetic variation in somatic human tissues. Proc Natl Acad Sci U S A, 109(44), 18018-18023. doi:10.1073/pnas.1213736109
- Paakki, P., Stockmann, H., Kantola, M., Wagner, P., Lauper, U., Huch, R., . . . Pasanen, M. (2000). Maternal drug abuse and human term placental xenobiotic and steroid metabolizing enzymes in vitro. Environ Health Perspect, 108(2), 141-145. doi:10.1289/ehp.00108141
- Strolin-Benedetti, M., Brogin, G., Bani, M., Oesch, F., & Hengstler, J. G. (1999). Association of cytochrome P450 induction with oxidative stress in vivo as evidenced by 3-hydroxylation of salicylate. Xenobiotica, 29(11), 1171-1180. doi:10.1080/004982599238038
- Malinda KM, Wysocki AB, Koblinski JE, Kleinman HK, Ponce ML. Angiogenic laminin-derived peptides stimulate wound healing. Int J Biochem Cell Biol. 2008;40(12):2771-80;
- Grinnell F, Billingham RE, Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981 Mar;76(3):181-9
- Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds. 2016 Mar;28(3):78-88
- Final Report: Evaluation of the host response to wound care products in a chronic wound model
- Data on file – TR-0103
- Gilpin A, Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int. 2017;2017:9831534. doi: 10.1155/2017/9831534. Epub 2017 Apr 30. PMID: 28540307; PMCID: PMC5429943
- Keane, T. J., Londono, R., Turner, N. J., & Badylak, S. F. (2012). Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials, 33(6), 1771- 1781. doi:10.1016/j.biomaterials.2011.10.054
- Sicari, B. M., Dziki, J. L., Siu, B. F., Medberry, C. J., Dearth, C. L., & Badylak, S. F. (2014). The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials, 35(30), 8605-8612. doi:10.1016/j. biomaterials.2014.06.060
- Hinton, J. P., Dvorak, K., Roberts, E., French, W. J., Grubbs, J. C., Cress, A. E., . . . Nagle, R. B. (2019). A Method to Reuse Archived H&E Stained Histology Slides for a Multiplex Protein Biomarker Analysis. Methods Protoc, 2(4). doi:10.3390/mps2040086









