InnovaMatrix® PD
Placental Extracellular Matrix
InnovaMatrix® PD is the first and only particulate placental ECM medical device cleared by the FDA for wound management.

About InnovaMatrix® PD
About InnovaMatrix® PD
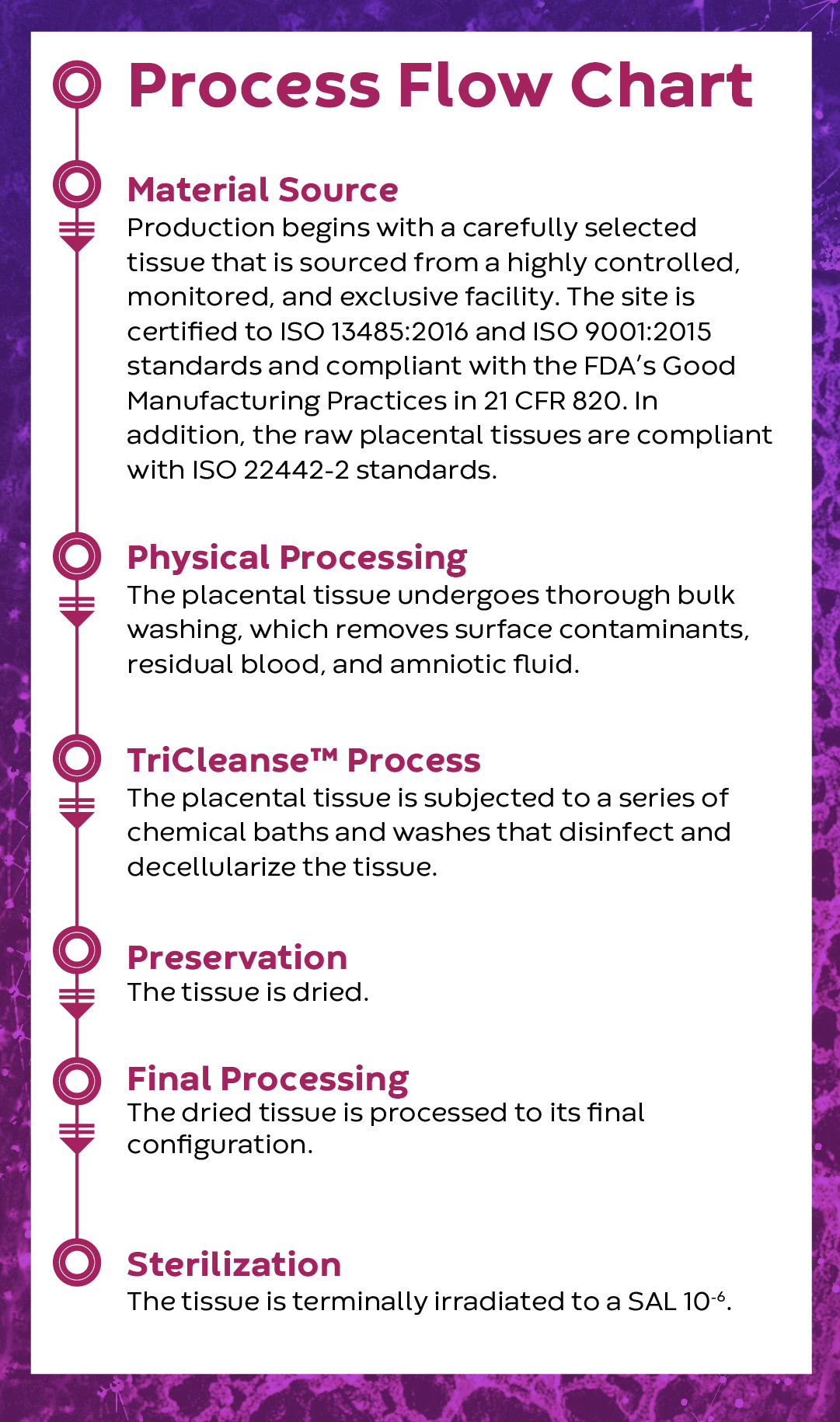
The introduction of InnovaMatrix® PD is an important and innovative advancement in the management of wounds. As a next-generation technology, InnovaMatrix® PD offers the inherent benefits of the placenta1,2 plus the quality control, reliability, and safety profile of a medical device3,4. Manufactured with our proprietary TriCleanse™ Placental Extracellular Matrix (ECM) Process, InnovaMatrix® PD is the next-generation particulate placental device designed for the management of complex surgical wounds, hard-to-heal wounds, and burns.


Indicated For the Management of Wounds Including:*
*See package insert for full list of indications.

Material Selection
While the benefits of placental-derived allografts are well documented1,2, human-derived grafts can be variable due to the unique medical histories and social behaviors of each donor5-12. Knowing this challenge, a porcine source was selected because it can be controlled for age, diet, health, and activity level, thereby reducing the variability of the raw material used to manufacture InnovaMatrix® PD.
The TriCleanse™ Process balances the need for decellularization while maintaining structural proteins of the ECM that help with healing13.
A Critical BalanceCleaner ECM promotes healing response14,15Effective decellularization removes cells and cellular debris while maintaining the structural proteins of the ECM that help with healing. |
 |
|---|

A Critical Balance
Cleaner ECM promotes healing response14,15
The presence of cells and/or cellular debris in biologic wound dressings can cause an inflammatory reaction resulting in a greater M1:M2 ratio macrophage response by the host immune system at the wound site. Thoroughly decellularized biologic skin scaffolds allow the host to respond directly with a healing response, generally associated with a greater M2:M1 ratio of macrophages and leading to collagen deposition14,15.

Indicated for the Management of Wounds Including:*
*See package insert for full list of indications.
- Partial and full-thickness wounds
- Pressure ulcers
- Venous ulcers
- Diabetic ulcers
- Chronic vascular ulcers
- Tunneled/undermined wounds
- Surgical wounds (donor sites/grafts, post-Mohs surgery, post- laser surgery, podiatric, wound dehiscence)
- Trauma wounds (abrasions, lacerations, and skin tears)
- Partial-thickness second degree burns
- Draining wounds
The device is intended for one-time use.
Contraindications
This device is derived from porcine collagen and should not be used on patients with sensitivity or allergy to porcine materials; sensitivity or allergy to collagen; or active or latent infection in or around the application site.
This device is not indicated for use in third degree burns.
Available Sizing
| Part # | Description | Size | SQ CM Surface Area |
| IMP-0100-01 | InnovaMatrix® PD | 100mg | *50 cm2 |
*Coverage Varies
Download our
InnovaMatrix® PD Documents
Use the arrows to navigate.
Contact Us
MKT-2023-0051-06E V01
- Shaifur Ra, M., Islam, R., Asaduzzama, S.M., & Shahedur R, M. (2015). Properties and Therapeutic Potential of Human Amniotic Membrane. Asian Journal of Dermatology, 7(1), 1-12. doi: 10.3923/ajd.2015.1.12
- Fairbairn, N. G., Randolph, M. A., & Redmond, R. W. (2014). The clinical applications of human amnion in plastic surgery. Journal of Plastic, Reconstructive & Aesthetic Surgery, 67(5), 662-675.
- K193552 510(k) Summary
- 7. Data on file -RDR-002
- Cardinal, L. J. (2015). Central tendency and variability in biological systems. J Community Hosp Intern Med Perspect, 5(3), 27930. doi:10.3402/jchimp.v5.27930
- Collier, A. C., Tingle, M. D., Paxton, J. W., Mitchell, M. D., & Keelan, J. A. (2002). Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum Reprod, 17(10), 2564-2572. doi:10.1093/humrep/17.10.2564
- DuBois, B. N., O’Tierney-Ginn, P., Pearson, J., Friedman, J. E., Thornburg, K., & Cherala, G. (2012). Maternal obesity alters feto-placental cytochrome P4501A1 activity. Placenta, 33(12), 1045-1051. doi:10.1016/j.placenta.2012.09.008
- Huuskonen, P., Amezaga, M. R., Bellingham, M., Jones, L. H., Storvik, M., Hakkinen, M., . . . Pasanen, M. (2016). The human placental proteome is affected by maternal smoking. Reprod Toxicol, 63, 22-31. doi:10.1016/j.reprotox.2016.05.009
- McRobie, D. J., Glover, D. D., & Tracy, T. S. (1998). Effects of gestational and overt diabetes on human placental cytochromes P450 and glutathione S-transferase. Drug Metab Dispos, 26(4), 367-371. Retrieved from https://www.ncbi.nlm. nih.gov/pubmed/9531526
- O’Huallachain, M., Karczewski, K. J., Weissman, S. M., Urban, A. E., & Snyder, M. P. (2012). Extensive genetic variation in somatic human tissues. Proc Natl Acad Sci U S A, 109(44), 18018-18023. doi:10.1073/pnas.1213736109
- Paakki, P., Stockmann, H., Kantola, M., Wagner, P., Lauper, U., Huch, R., . . . Pasanen, M. (2000). Maternal drug abuse and human term placental xenobiotic and steroid metabolizing enzymes in vitro. Environ Health Perspect, 108(2), 141-145. doi:10.1289/ehp.00108141
- Strolin-Benedetti, M., Brogin, G., Bani, M., Oesch, F., & Hengstler, J. G. (1999). Association of cytochrome P450 induction with oxidative stress in vivo as evidenced by 3-hydroxylation of salicylate. Xenobiotica, 29(11), 1171-1180. doi:10.1080/004982599238038
- Gilpin A, Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int. 2017;2017:9831534. doi: 10.1155/2017/9831534. Epub 2017 Apr 30. PMID: 28540307; PMCID: PMC5429943
- Keane, T. J., Londono, R., Turner, N. J., & Badylak, S. F. (2012). Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials, 33(6), 1771- 1781. doi:10.1016/j.biomaterials.2011.10.054
- Sicari, B. M., Dziki, J. L., Siu, B. F., Medberry, C. J., Dearth, C. L., & Badylak, S. F. (2014). The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials, 35(30), 8605-8612. doi:10.1016/j. biomaterials.2014.06.060







